Prompt administration of empiric IV antibiotics is the cornerstone of effective sepsis therapy. Multiple studies have shown that a delay in antibiotic administration as short as 1 hour after symptom onset can negatively impact survival, with an increase in mortality of 3-7% for every hour of delay. (1, 2, 3) Consequently, international sepsis guidelines such as those from the Surviving Sepsis Campaign (SSC) recommend prompt administration of IV empiric broad-spectrum antibiotics within as little as 1 hour of sepsis diagnosis.
The push to give empiric broad-spectrum antibiotics faster in potentially septic patients has caused concern in some circles that this effort may lead to the overprescribing of these potent antibiotics and contribute to antimicrobial resistance. (4, 5) Antibiotic resistance, as is well known, is a growing global health crisis that threatens to render many essential antibiotics useless in the face of emerging superbugs. (6) For this reason, sepsis guidelines generally emphasize the importance of antibiotic stewardship including obtaining appropriate cultures prior to antibiotic administration and narrowing the initial broad-spectrum empiric antibiotics to appropriately targeted antimicrobials as soon as culture and antibiogram results return, or is otherwise feasible. However, cultures can take several days to return results and in many cases these return negative, so it is unclear how often empiric antibiotics are narrowed and when this narrowing occurs.
To further investigate this issue and other trends in the use of antibacterial antibiotics for the treatment of sepsis, we conducted a survey of our Sepsis Clinical Guide app users between April 5th and May 10th, 2019. This survey examined various aspects of antibiotic therapy in sepsis including the use of cultures and rapid diagnostic tests, the types, number and duration of empiric antibiotics used and their de-escalation patterns, and the prevalence of multi-drug resistant organisms. This article presents the results of this survey and briefly discusses the implications of its findings.
Antibiotic Survey Results
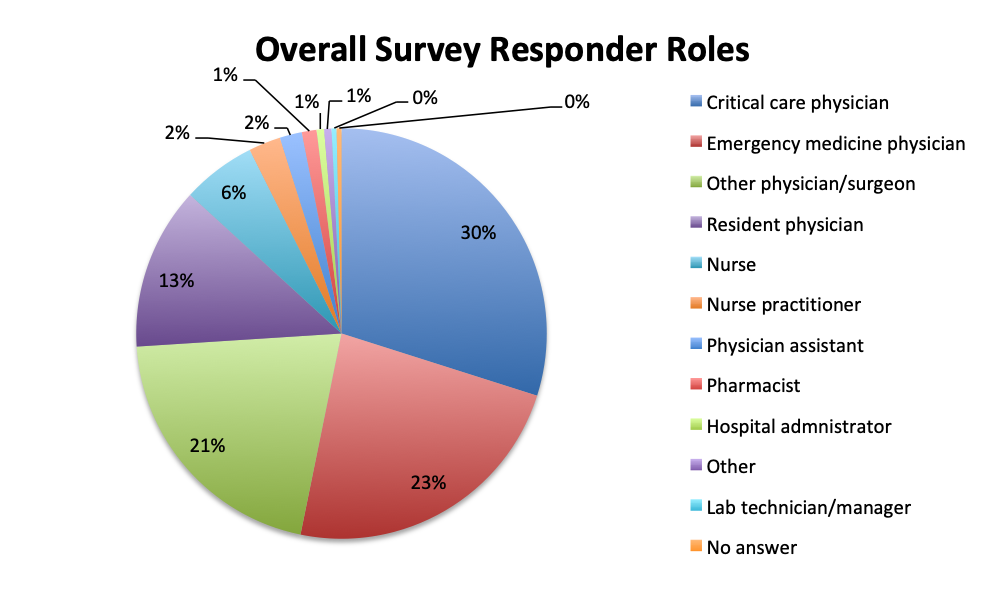
A total of 515 sepsis app users completed our antibiotic survey, 265 respondents in the English version and 250 in the Spanish version of our app. Spanish language survey respondents represented primarily Latin America (except Brazil) and Spain, whereas English language survey respondents were from North America, Brazil, Europe and other regions around the world. Among all respondents, 87% were physicians, practicing primarily in the fields of critical care (30%) and emergency medicine (23%), 10% were nurses, nurse practitioners or physician assistants, and the remaining 3% included other healthcare professionals such as hospital administrators, pharmacists, and lab technicians.
The sections that follow summarize the survey questions and responses overall and individually for the English and Spanish versions to highlight differences in management between the geographical areas covered by these two versions.
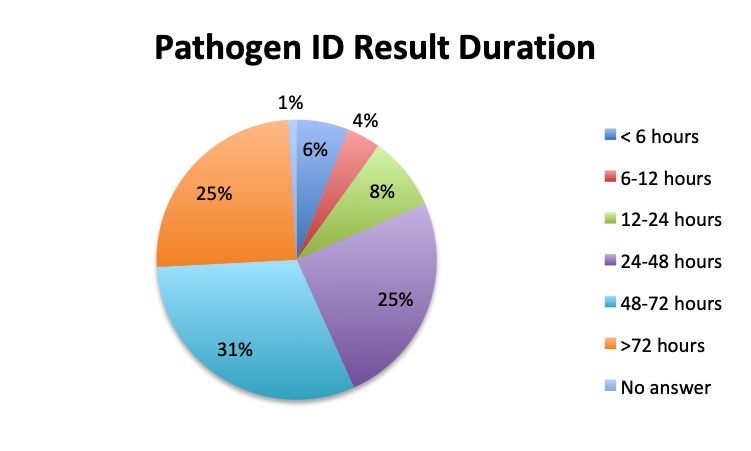
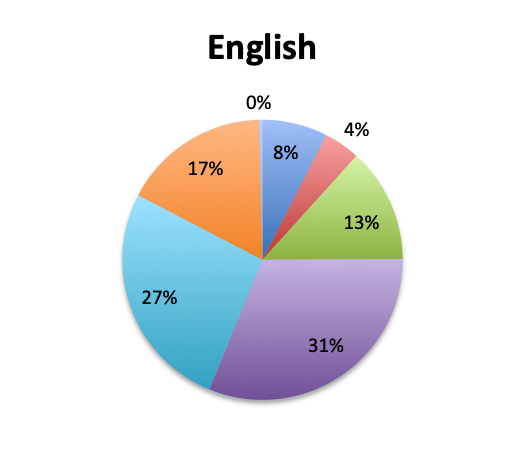
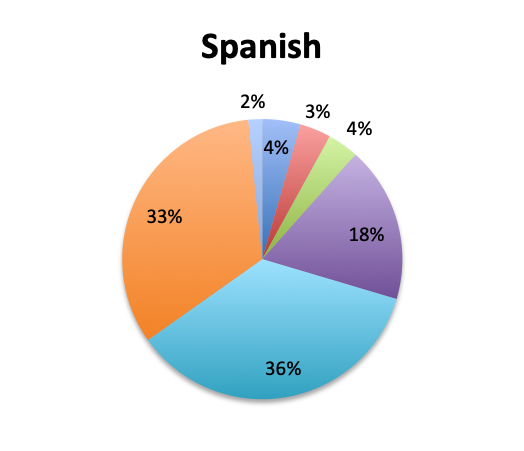
Question 1. How long does it typically take to obtain results for pathogen identification tests (eg, cultures or other tests) at your institution?
A. Less than 6 hours
B. 6-12 hours
C. 12-24 hours
D. 24-48 hours
E. 48-72 hours
F. More than 72 hours
 |
|
 |
 |
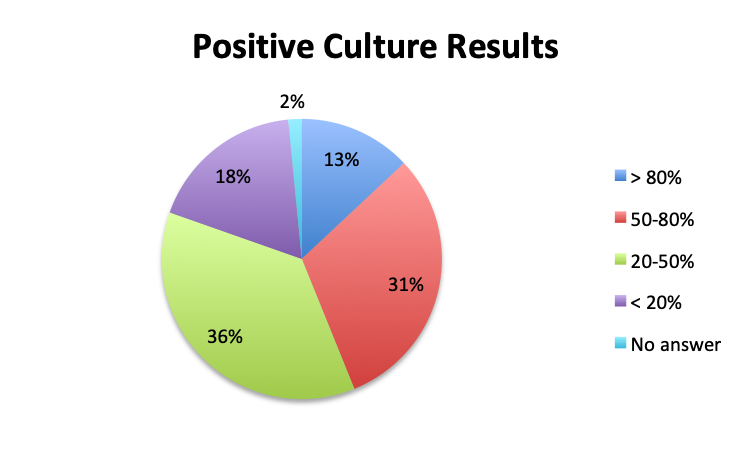
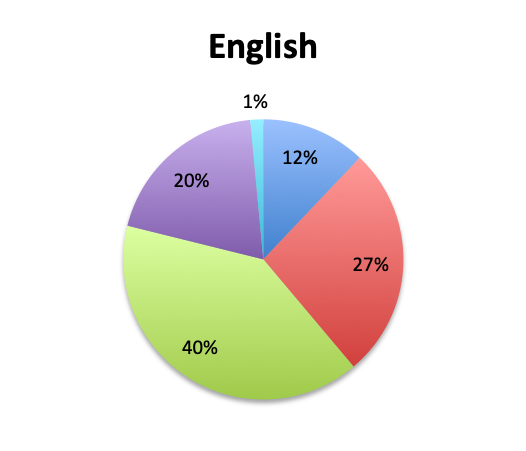
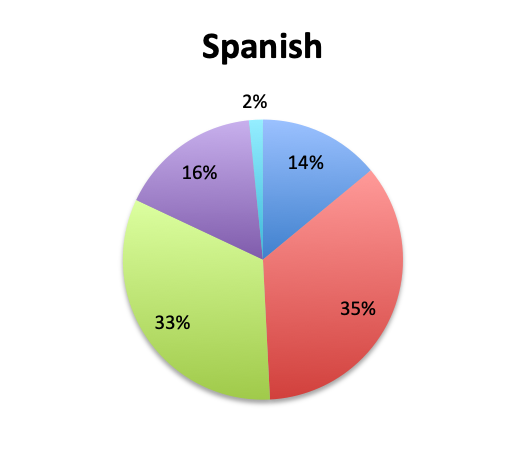
Question 2. In your clinical experience, in what percentage of patients treated for sepsis do initial cultures return positive?
A. More than 80%
B. 50-80%
C. 20-50%
D. Less than 20%
 |
|
 |
 |
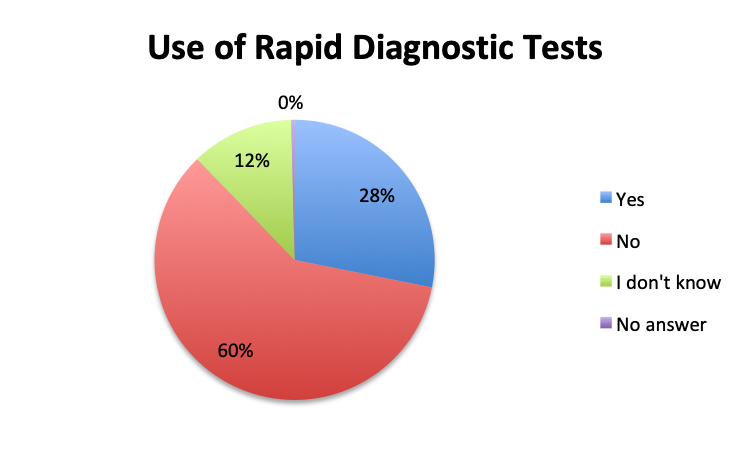
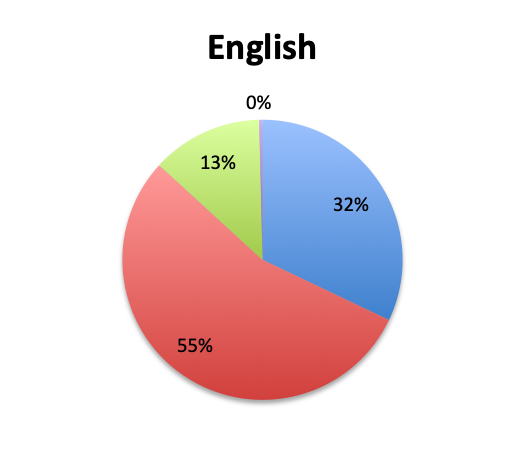
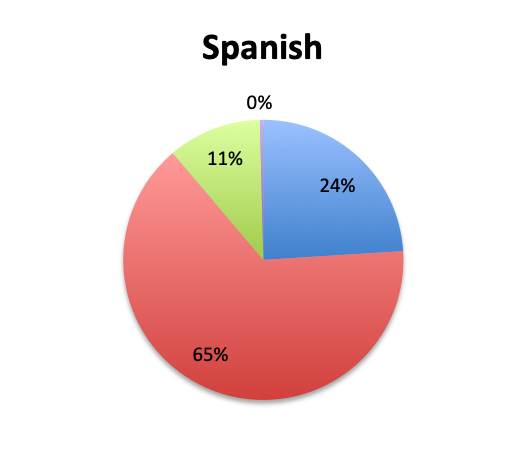
Question 3. Rapid diagnostic tests (RDTs) use various technologies such as PCR or FISH to identify infectious pathogens much faster than traditional cultures, sometimes in as little as 1 hour. Are RDTs available to you at your institution?
A. Yes
B. No
C. I don’t know
 |
|
 |
 |
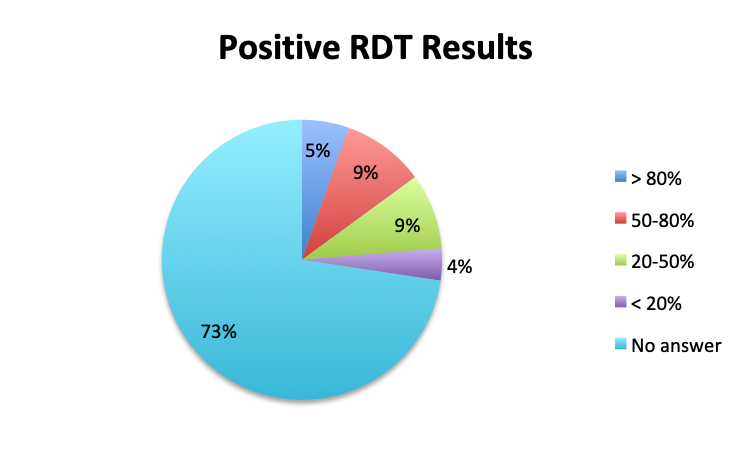
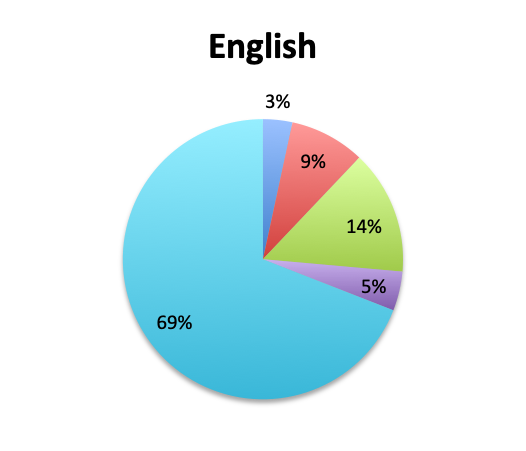
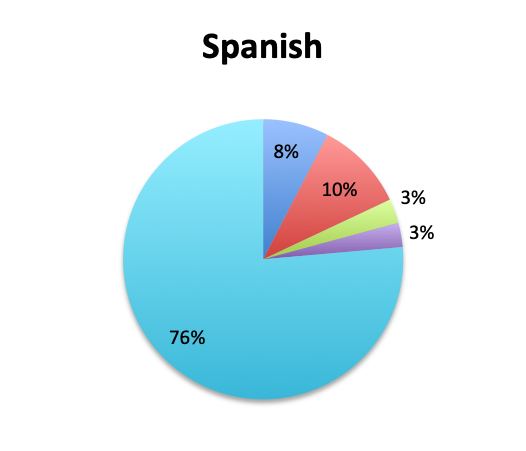
Question 4. How often do Rapid Diagnostic Tests (RDT) return positive in patients treated for sepsis, in your experience?
A. More than 80%
B. 50-80%
C. 20-50%
D. Less than 20%
 |
|
 |
 |
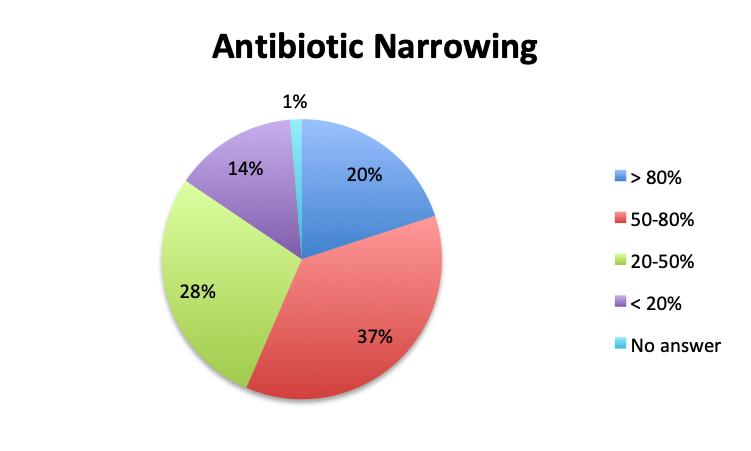
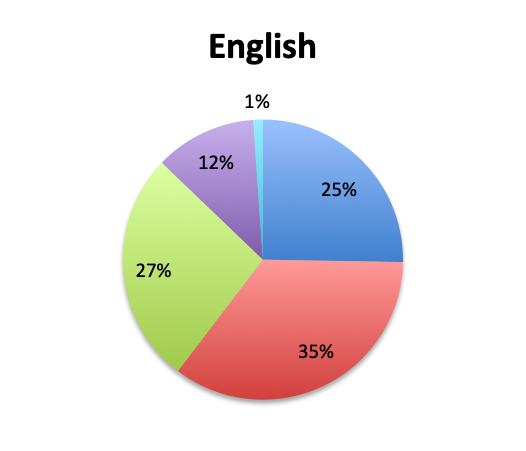
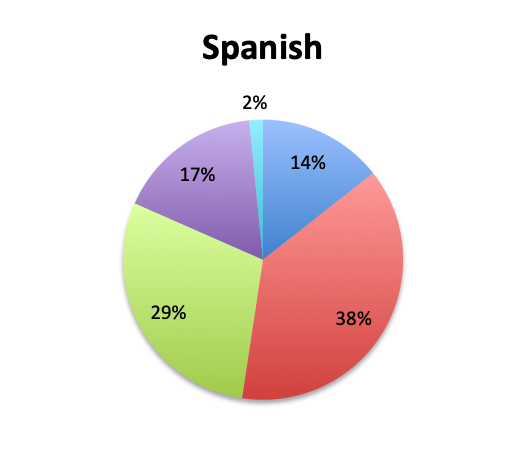
Question 5. On average, in what percentage of septic patients do you adjust or narrow your antibiotic regimen over the course of treatment?
A. More than 80%
B. 50-80%
C. 20-50%
D. Less than 20%
 |
|
 |
 |
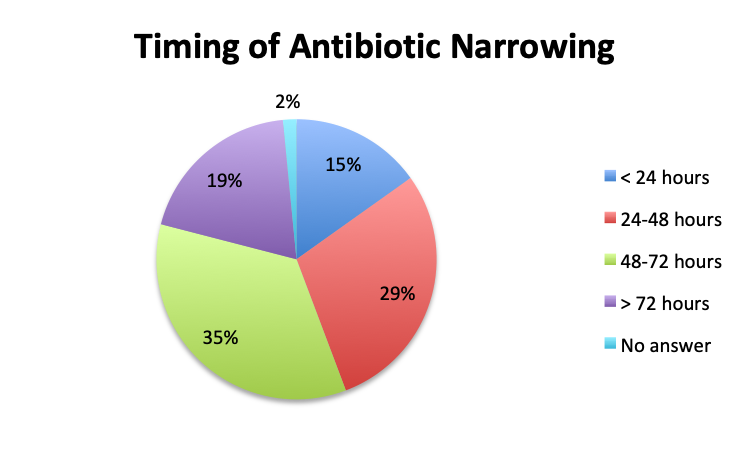
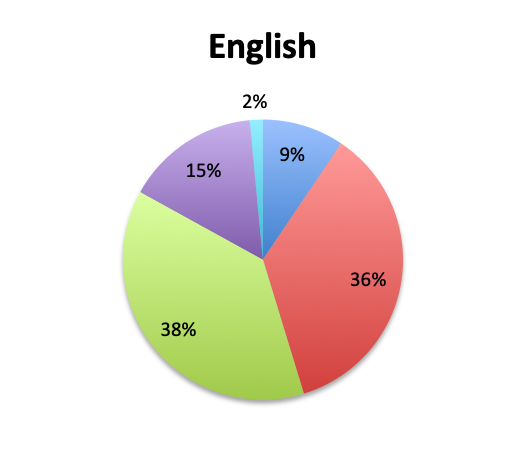
Question 6. On average, in patients in whom you adjust your antibiotic regimen, approximately how many hours after the initial administration of empiric antibiotics do you make the adjustment?
A. Less than 24 hours
B. 24-48 hours
C. 48-72 hours
D. More than 72 hours
 |
|
 |
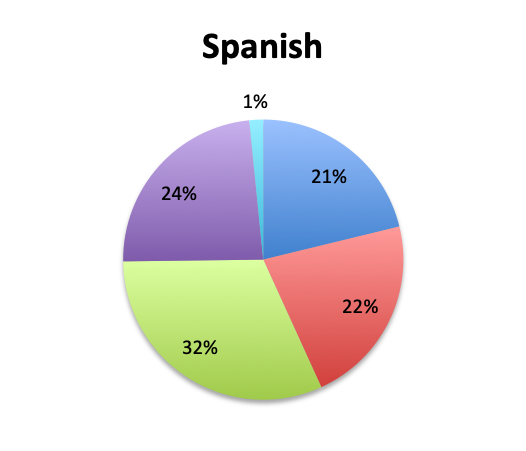 |
| Note: Of note, in the Spanish survey, the <24 hours choice was divided into 3 separate choices which included <6 hours, 6-12 hours, and 12-24 hours. The “<24 hours” total shown here for the Spanish survey is a sum of those 3 choices. | |
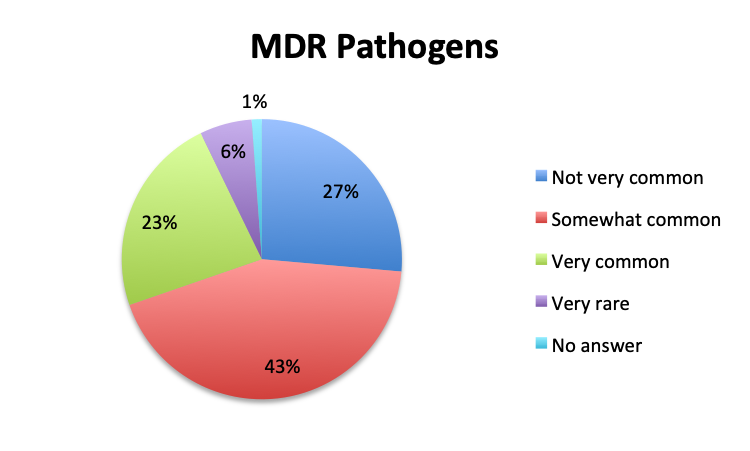
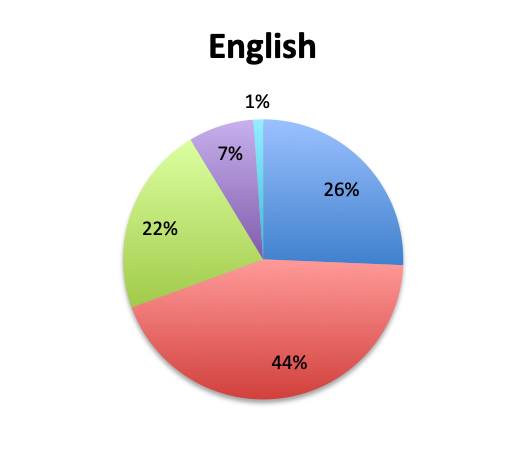
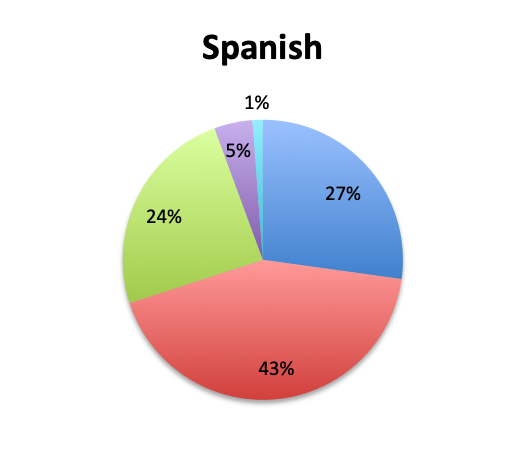
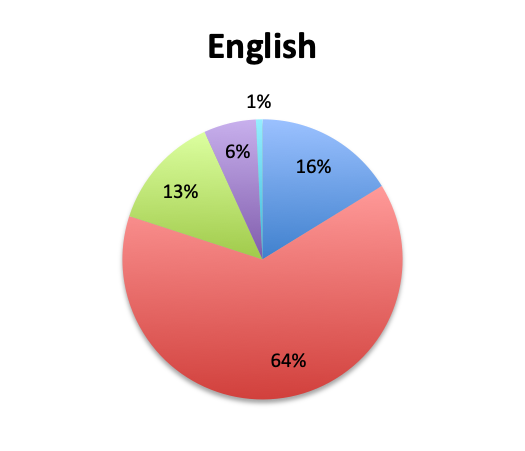
Question 7. How common are infections with multi-drug-resistant pathogens at your institution?
A. Very common
B. Somewhat common
C. Not very common
D. Very rare
 |
|
 |
 |
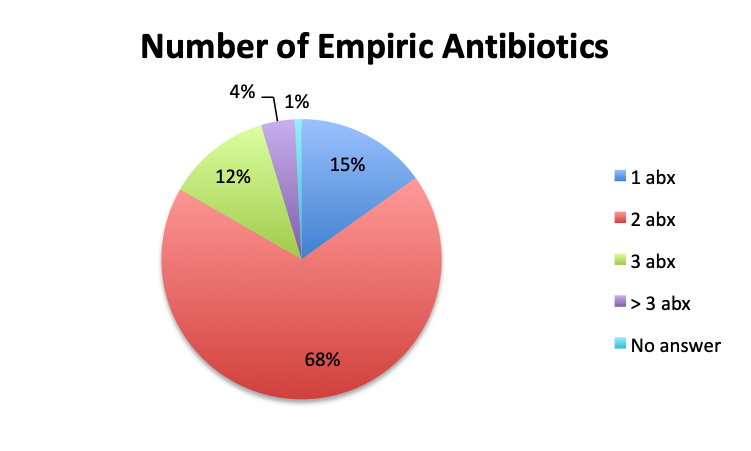
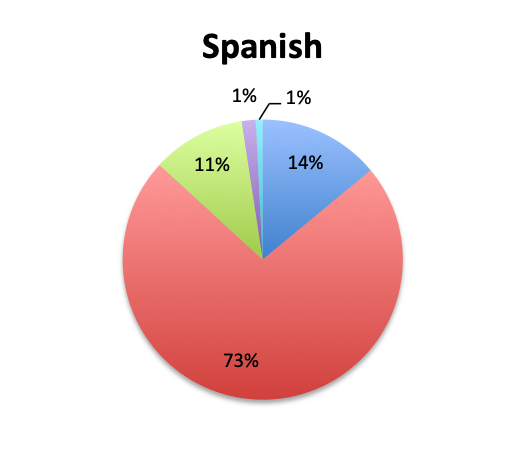
Question 8. On average, how many IV empiric antibiotics does a patient diagnosed with sepsis initially receive at your institution?
A. 1
B. 2
C. 3
D. More than 3
 |
|
 |
 |
Question 9. What is the average length of IV antibiotic therapy for sepsis in your clinical experience? (this question was omitted from the Spanish survey)
A. Less than 3 days
B. 3 to 5 days
C. 5 to 7 days
D. 7 to 10 days
E. More than 10 days
 |
Question 10. Which two among the following antibiotic classes do you most commonly use in your initial empiric treatment of sepsis? (select only the top 2)
A. β-lactam/β-lactamase inhibitor (eg, piperacillin-tazobactam)
B. 3rd/4th generation cephalosporin (eg, ceftriaxone, cefepime)
C. Carbapenem (eg, meropenem, imipenem)
D. Monobactam (eg, aztreonam)
E. Fluoroquinolone (eg, ciprofloxacin)
F. Macrolide (eg, azithromycin)
G. Aminoglycoside (eg, gentamicin, amikacin)
H. Other empiric antibiotics [user entry]
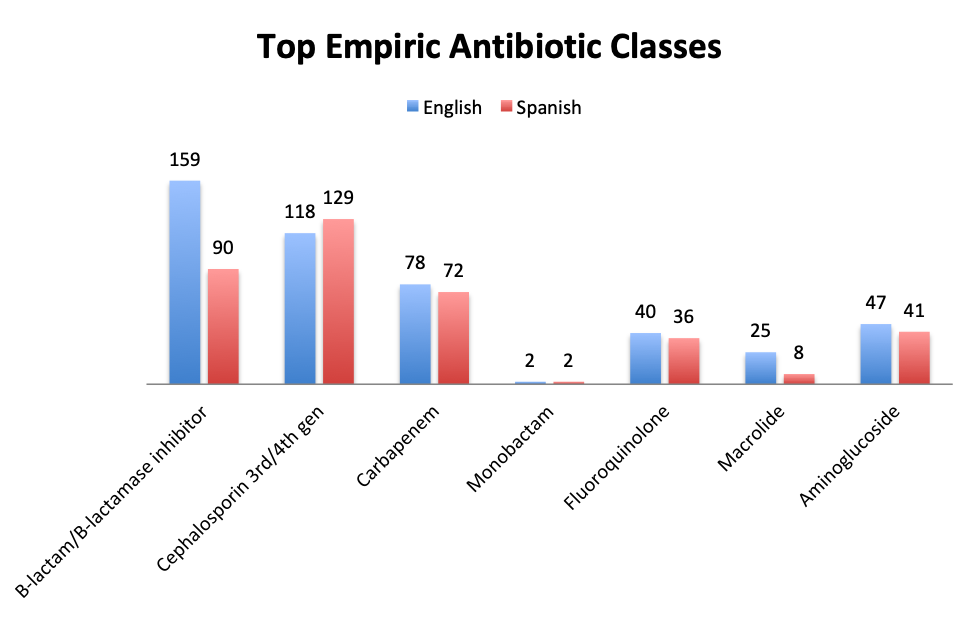 |
| Note: Although users were asked to select only 2 of the antibiotic classes in the answer choices, a number of respond selected only 1 or more than 2. The numbers shown represent the totals for each antibiotic class. The most popular combinations were β-lactam and cephalosporin, and β-lactam and carbapenem. Other user-entered antibiotics included metronidazole and vancomycin (which is covered in question 11). |
Question 11. Which of the following antibiotics do you most commonly use to empirically cover potentially resistant Gram-positive bacteria (eg, MRSA) in sepsis:
A. Vancomycin
B. Daptomycin
C. Linezolid
D. Clindamycin
E. Teicoplanin
F. Tigecycline
G. Other [user entry]
 |
|
 |
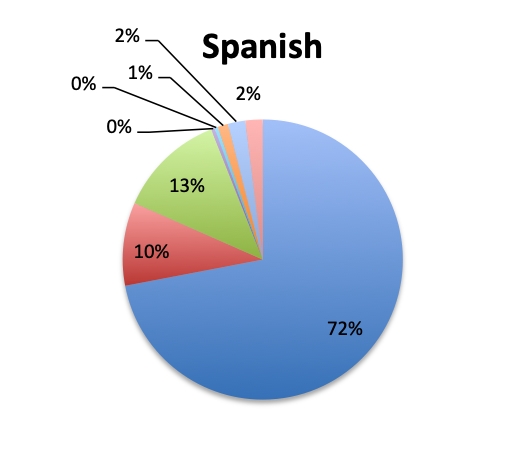 |
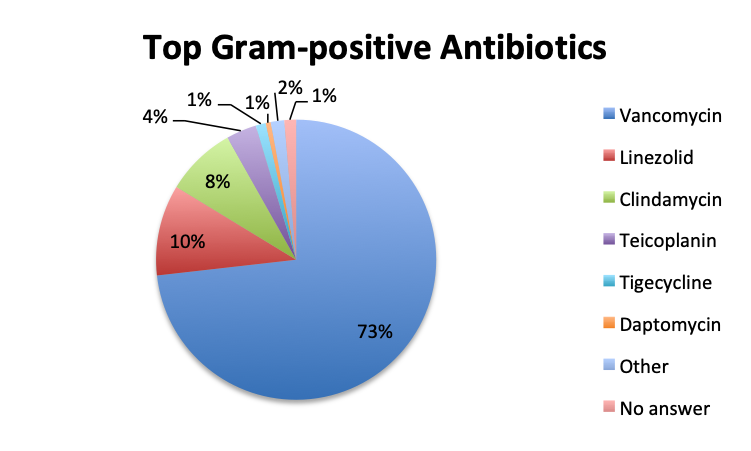
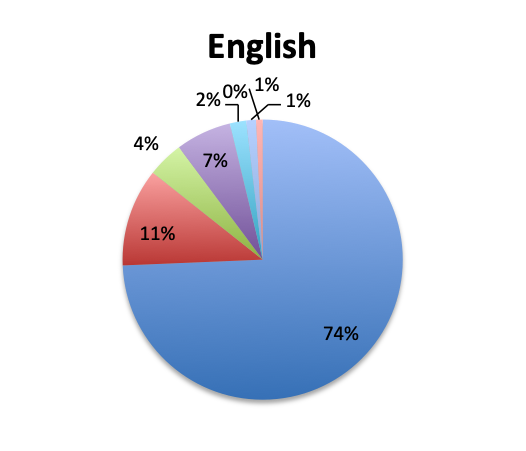
| Note: For this question, users could select only one of the available Gram-positive antibiotic choices so the choices are represented as percentages of the total responses (515 total, 265 in the English survey and 250 in the Spanish survey). | |
Question 12. Please enter any additional comments you may have on antibiotic treatment in sepsis (e.g. preferred empiric antibiotic regimen, recommendations, comments on antibiotic resistance or antibiotic needs, etc.)
| Additional Respondents Comments – Spanish Survey | |
|
|
| Note: This question was included only in the Spanish survey. User responses have been translated and revised for language. Words added for clarification are indicated in brackets. |
Question 13. Please select the choice that best describes your role:
| English survey options: A. Emergency medicine physician B. Critical care physician C. Other physician D. Nurse E. Nurse practitioner F. Physician assistant G. Resident physician/intern H. Pharmacist I. Hospital administrator J. Other [user entry] |
Spanish survey options: A. Emergency medicine physician B. Critical care physician C. Other physician D. Nurse E. Pharmacist F. Resident physician G. Other [user entry] |
 |
 |
 |
Participating Countries
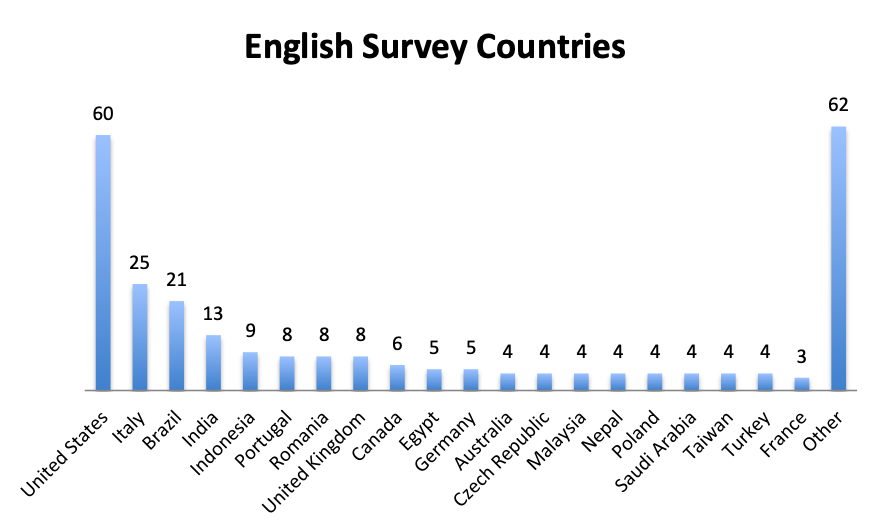 |
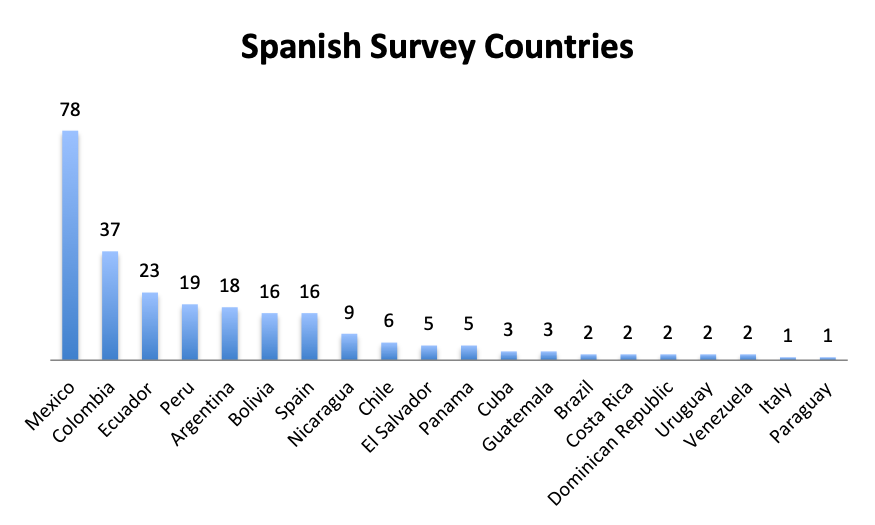 |
| Note: A total of 59 countries participated in the English survey and 20 countries in the Spanish survey (all shown). The “Other” category in the English Survey Countries chart represents 62 responses from 40 countries. |
Results Summary
Our survey provided a number of insights on the use of antibiotics in sepsis and demonstrated significant variations in their management among different regions of the world. These are summarized below.
Culture effectiveness and duration
Not surprisingly, a majority of survey respondents (55%) indicated that pathogen identification typically takes 2 days or longer, and 25% reported that identification takes 3 days or more. Spanish survey respondents reported significantly longer durations, with 69% reporting that identification takes 2 days or longer vs only 43% in the English survey. Notably, almost twice as many respondents in the Spanish survey indicated that pathogen identification takes longer than 3 days (33% vs. 17%).
Looking at the effectiveness of cultures in identifying pathogens, overall, only 13% of respondents answered that cultures and other identification return positive >80% of the time, while the majority of respondents (67%) reported positive results 20-80% of the time, and 18% reported positive results less than 20% of the time. The distribution of responses suggests that overall, cultures return positive somewhere in the 50% range. Interestingly, the rate of positive culture results seemed to be somewhat higher in the Spanish survey, perhaps because culture durations were also longer in this survey.
Rapid diagnostic testing
Rapid Diagnostic Tests (RDTs) were not reported to be widely available, and they were significantly less so in the Spanish survey, with only 24% of Spanish survey respondents reporting RDT availability vs 32% of English survey responders. Among those using RDTs, the response pattern suggested that on average RDTs also return positive about half the time overall, a rate similar to that of cultures. Interestingly, however, considerably more Spanish survey respondents reported a >50% rate of positive results with these tests than English survey respondents (75% vs. 39% in the English survey among those using these tests), although the significance of this difference is uncertain given the small sample.
Antibiotic Narrowing
Overall, more than half of respondents (57%) reported narrowing empiric antibiotics over the course of sepsis treatment in more than 50% of the patients they treat, with this percentage being somewhat lower in the Spanish survey (52%) than the English survey (60%). Overall, the majority of respondents (64%) reported narrowing antibiotics between 24 and 72 hours after initial administration, with only 15% reporting narrowing before 24 hours.
Interestingly, more respondents reported narrowing antibiotics sooner than 24 hours in the Spanish survey, which is counterintuitive given the slower culture results in this survey. However, it should be noted that the Spanish survey had 3 early interval choices (<6 hours, 6-12 hours, 12-24 hours) vs. only 1 such choice in the English survey (<24 hours), potentially causing a bias for these choices and thus a higher than expected <24 hours total. The higher number of Spanish survey respondents reporting narrowing of antibiotics at >72 hours (24% vs. 15%) seems to indicate that antibiotic narrowing does in fact occur later among Spanish survey responders, which is consistent with the slower culture results found in this survey.
Prevalence of multi-drug resistant bacteria
We also qualitatively assessed the prevalence of multi-drug-resistant (MDR) pathogens in our survey, and not surprisingly these were found to be fairly common. Overall, 24% of responders reported MDR organisms being “very common,” while 43% reported them being “somewhat common.” Notably, there was very little difference between the English and Spanish surveys with regard to MDR prevalence. The sample size was too small to draw meaningful conclusions at the country level for most countries, but a higher percentage of “very common” responses were seen in Argentina (8 of 18; 44%) and Mexico (25 of 77; 32%) in the Spanish survey, and in Indonesia (5 of 9; 56%), India (4 of 13; 31%) and Romania (3 of 8; 38%) in the English survey. In the US, only 6 of 60 responders (10%) reported MDR organisms to be “very common.”
Antibiotic use
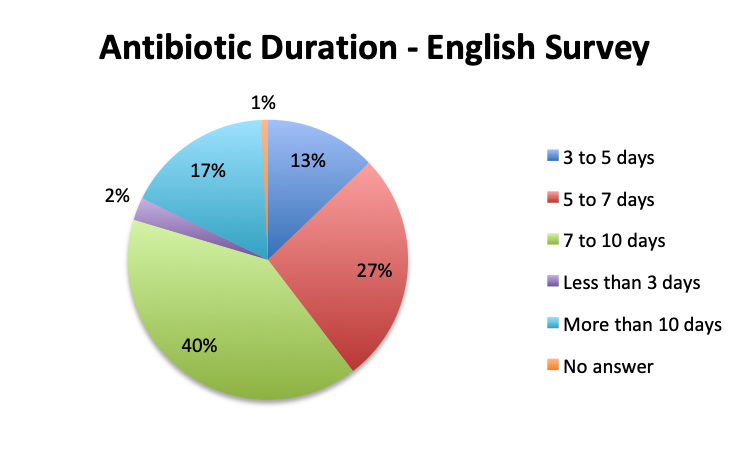
The large majority of respondents (68%) reported starting antibiotic therapy with 2 initial empiric antibiotics, which is largely in line with treatment guideline recommendations in the absence of suspicion of MDR organisms. Interestingly, six times more respondents in the English survey than the Spanish survey (6% vs. 1%) indicated using more than 3 empiric antibiotics, potentially indicating more liberal antibiotic prescribing practices or a sicker patient population in this sample, which is possible since the English survey had a higher percentage of critical care physicians. The average duration of therapy was only recorded in the English survey and this was reported to be 5 to 10 days by the majority of respondents (67%). Significantly fewer respondents reported using short (5 days or less; 15%) or long (>10 day; 17%) regimens.
Not surprisingly, β-lactams with β-lactamase inhibitors such as piperacillin-tazobactam, and 3rd/4th generation cephalosporins such as ceftriaxone were among the most frequently used antibiotic classes in sepsis treatment. Vancomycin was by far (73%) the top choice for Gram-positive coverage when MDR bacteria are suspected, with linezolid in a distant second place at 10%. Clindamycin was in third place and significantly more preferred in the Spanish than the English survey (13% vs 4%), whereas use of the newer agents like teicoplanin and tigecycline was infrequently reported overall.
There were, however, a few interesting exceptions to these findings, namely, teicoplanin was by far favored over vancomycin in the UK (6 of 8; 75%), and was also somewhat popular in Brazil (4 of 21; 19%), whereas Spain strongly preferred linezolid (10 of 16; 63%) as did Italy to a lesser degree (9 of 25; 36%). Mexico and the US, the survey’s top responders, strongly preferred vancomycin (almost completely in the US, 54 of 60; 90%).
In the free-text comment section of the survey, which was only available for the Spanish language survey, a number of users noted their preferred antibiotic regimens in different clinical scenarios. Most regimens included a combination of a Gram-positive agent such as clindamycin with Gram-negative or broader spectrum agents such as ceftriaxone or gentamicin. One user indicated the use of metronidazole in combination with gentamicin or ceftriaxone. We should note that metronidazole would be an appropriate empiric antibiotic in sepsis if anaerobic sepsis (eg, GI source) was suspected. Also noteworthy was a comment indicating rising incidence of ESBL-producing E. Coli due to overuse of cephalosporins like ceftriaxone and quinolones like ciprofloxacin. Several users indicated the importance of cultures, antibiograms, and rapid diagnostic testing, while one user surprisingly noted that in his or her institution treatment does not rely on cultures at all.
Survey demographics
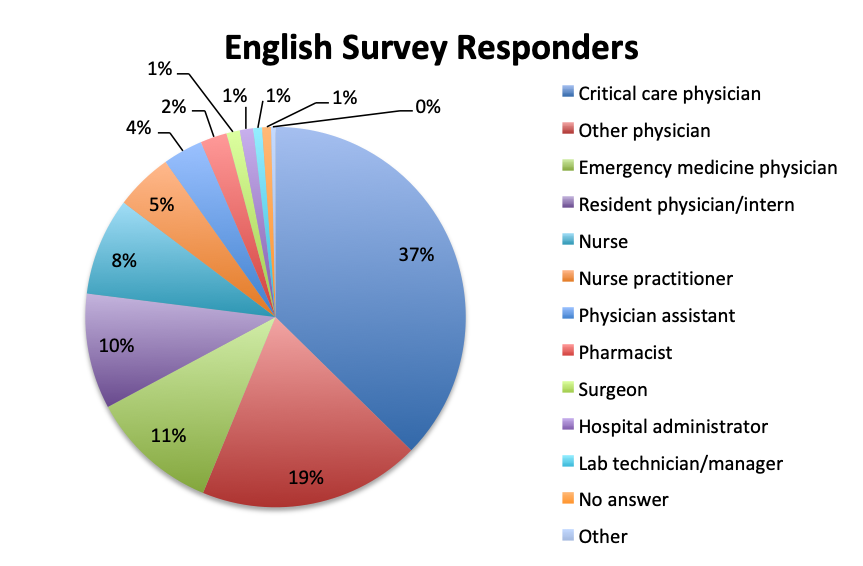
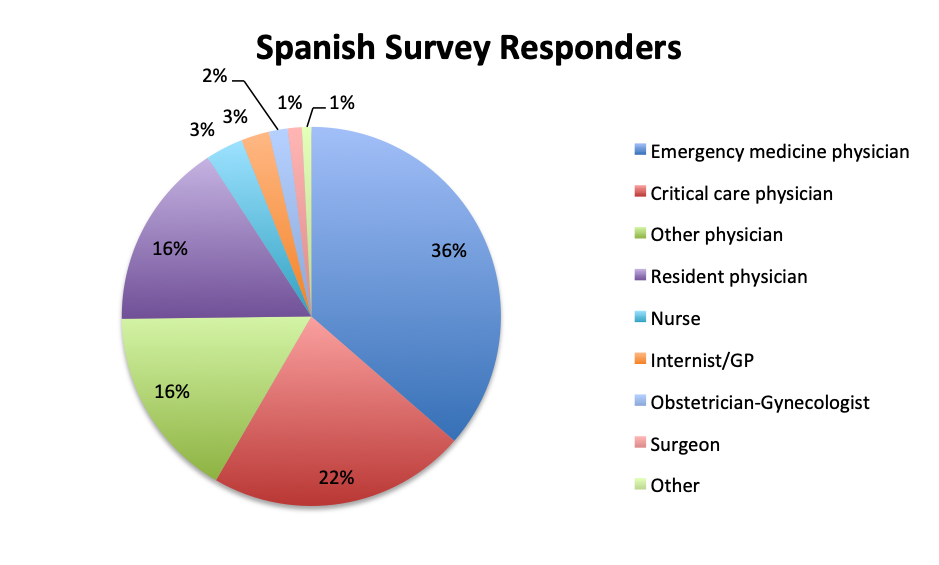
The large majority of overall respondents were physicians (87%), with this percentage being significantly higher in the Spanish language survey than in the English survey (96% vs. 78%). Overall, 30% of physicians answering the survey were critical care practitioners, with emergency medicine physicians in second place at 23%. This was driven primarily by the English survey where most responders (37%) were critical care physicians, whereas in the Spanish survey, most responders were emergency medicine physicians (36%). In the English survey, nurses contributed considerably more to the survey than in the Spanish survey (8% vs. 3%), as did a number of other healthcare professionals such nurse practitioners, physicians assistants, pharmacists, lab technicians and administrators (13%).
Responders came from 59 countries in the English survey, distributed throughout the world, and from 20 countries in the Spanish survey, primarily in Latin America and Spain. Mexico and the US were the top responders, with 78 and 60 responders, respectively, followed by Columbia and Italy with 37 and 25 responders.
Discussion
One of the key objectives of our survey was to shed some light on antibiotic stewardship practices in the treatment of sepsis, including the use and effectiveness of pathogen identification testing, the number and types of empiric antibiotics given, and the timing of their de-escalation. Results suggested that while empiric antibiotics are narrowed in many patients, perhaps in as many as 50 to 60%, de-escalation is constrained by limitations in current pathogen identification methods.
Cultures were reported to take 2-3 days or longer to return results in the large majority of cases (81%), and results positively identified the infectious agent only about half the time. Furthermore, new rapid diagnostic testing (RDT) systems were not found to be in wide use, with only about a third of respondents reporting using them. These systems use PCR DNA amplification, peptide nucleic acid fluorescence in situ hybridization (PNA FISH) or other techniques to significantly reduce the time to pathogen identification, and in some cases even provide antibiotic susceptibility, from days to mere hours, making possible faster antibiotic de-escalation.(7) Latin American countries, in particular, reported lower use of RDTs and longer times to pathogen identification, something that was reflected in the lower rates of antibiotic de-escalation and longer intervals to de-escalation.
We should, however, point out that while pathogen identification rates and timing clearly affected antibiotic de-escalation rates and timing, we found that antibiotic narrowing could not be explained by pathogen identification alone. Whereas only 44% of respondents reported positive cultures more than 50% of the time, 57% of respondents reported narrowing antibiotics in more than 50% of patients. This suggests that other factors are involved in the decision to narrow antibiotics, which might include source identification, clinical assessment, and consideration of local pathogen prevalence and antibiotic resistance patterns.
It is also interesting to note that there were wide variations in the perceived efficacy of culture results, with 13% of respondents indicating cultures return positive >80% of the time and 18% that they return positive <20% of the time. This disparity suggests local factors play a role in culture effectiveness, for example, culture technique or laboratory capabilities. It is crucial that appropriate culture technique be followed in all cases, namely that two sets of two 15 mL blood culture samples (aerobic and anaerobic) each be drawn, and that these samples include blood from indwelling catheters if any are in place. Cultures should also be done on samples of other suspected sources including urine, sputum, wounds, surgical sites or any other suspect bodily fluids.
The majority of respondents reported administering 2 empiric antibiotics, which is in line with prevailing treatment guidelines. In general, the decision to administer more than 2 antibiotics, typically 3, rests on suspicion of MDR organisms, and in particular Pseudomonas aeruginosa, which is a prevalent and often resistant and highly pathogenic bacteria in institutional and especially critical care settings. (8) Overall, 15% percent of respondents reported administering only 1 empiric antibiotic. We should note that this should typically only be done in specific cases where the source is known and a particular pathogen class is highly suspected and not thought to be MDR. Conversely, 6% of English survey respondents reported using >3 antibiotics. Use of 4 or more antibiotics should not generally be necessary unless the patient is also suspected of viral or fungal sepsis, or infection with MDR organisms.
MDR organisms were reported to be common in our survey, and about a quarter of respondents overall reported that they are “very common,” a rather worrisome finding. Unfortunately, the sample size was too small for most countries to determine country-specific MDR prevalence patterns, but certain trends were apparent in a few cases, as mentioned in the Results Summary section. These trends were most apparent in the US and Mexico, the 2 countries with the most respondents and highest sample sizes, where three times as many people reported MDR pathogens being “very common” in Mexico vs. the US (32% vs. 10%). We did not find any country-level correlation between use of antibiotics, either number or classes, and MDR rates, but sample sizes were too small for most countries to draw meaningful conclusions.
Not surprisingly, β-lactams+β-lactamase inhibitors, 3rd and 4th generation cephalosporins, and carbapenems were among the leading classes of antibiotics used in the treatment of sepsis. Although these are broad spectrum antibiotics with Gram-positive and Gram-negative coverage, they have spotty Gram-positive coverage, and most importantly, they do not cover MRSA. As such, a second antibiotic with strong Gram-positive coverage is usually required in empiric sepsis treatment, and this is commonly vancomycin, as strongly demonstrated in our survey. Vancomycin resistant enterococci (VRE) are fairly common and may pose a problem in typical double-antibiotic vancomycin-based regimens if the cocci also happen to also be resistant to the second agent, such as a piperacillin-tazobactam, a commonly used β-lactam/β-lactamase inhibitor. In such cases, a VRE active antibiotic such as linezolid, daptomycin, tigecycline or quinupristin-dalfopristin should be considered. Common sources for community-acquired enterococcal bacteremia are the GI and GU tracts, while nosocomial sources commonly include intravascular or urinary catheters, but also intra-abdominal, burn wounds, pelvic, biliary, and bone sources. (9)
Thirteen percent of Spanish survey respondents reported using clindamycin for Gram-positive coverage when infection with MDR organisms is suspected. We should note that a significant fraction of MRSA can exhibit both constitutive and inducible resistance to clindamycin. One study found 53.4% of isolates to have constitutive resistance and 20.5% to have inducible resistance, but these resistance rates vary widely across geographical regions. (10) MRSA strains with inducible clindamycin resistance (iCR) show sensitivity on routine testing, but have a positive D-test. A D-test should therefore be done to test for inducible resistance whenever clindamycin is considered for MRSA treatment to prevent subsequent treatment failure. (10) Furthermore, clindamycin does not provide Enterococcal coverage and should not be used when these are possible suspects (eg, GI, GU sepsis) unless a second agent covers them.
Pseudomonas is one of the other MDR threats on the Gram-negative end of the spectrum, and should be highly suspected in immunocompromised and critically ill patients, and especially those who are or have recently been on a ventilator. Although the top three antibiotic classes with Gram-negative coverage mentioned above (β-lactam/β-lactamase inhibitors, cephalosporins and carbapenems) all have Pseudomonal coverage, Pseudomonas has a wide range of resistance patterns and can be resistant to one, two or all of these antibiotic classes. (11) For this reason, as previously mentioned, whenever Pseudomonal sepsis is suspected, a triple antibiotic regimen is typically required: vancomycin or another broad spectrum Gram-positive antibiotic like linezolid plus two Gram-negative antibiotics from the 3 Gram-negative classes above, or one of these classes plus another anti-pseudomonal antibiotic like gentamicin, ciprofloxacin or aztreonam.
One should also keep in mind the emerging MDR threat of extended-spectrum beta-lactamase (ESBL) producing organisms, such as ESBL strains of Klebsiela pneumoniae and E. coli, which render β-lactams and traditional cephalosporins useless. For these infections, carbapenems, new caphalosporin/carbapenem-β-lactamase-inhibitor combination drugs like ceftolozane-tazobactam or merepenem-vaborbactam, or tigecycline are required. For the dreaded carbapenem resistant Enterobacteriaceae (CRE), the antibiotic options narrow drastically to aminoglycosides like gentamicin, tigecycline, or as a last resort, colistin.
To conclude, the key finding of our survey was that while many clinicians de-escalate empiric antibiotics during the treatment of sepsis, de-escalation occurs only in about half the patients and its timing is constrained by slow methods of pathogen identification. Although rapid diagnostic testing methods that reduce pathogen identification times, and even antibiotic susceptibility test times, from days to mere hours are currently available, these appear not to be in wide use. Wider adoption of these tests is an important component of antibiotic stewardship and should be a high-priority goal for all healthcare systems. Empiric sepsis treatment requires the use of multiple potent broad-spectrum antibiotics which should be used judiciously and no longer than necessary to reduce the development of antibiotic resistance. Multi-drug resistant organisms are a growing global threat which must be combatted through good antibiotic stewardship.
Conclusion
In our survey of 515 Sepsis Clinical Guide app users, primarily physicians in critical care, emergency medicine and other specialties, but also nurses and other healthcare professionals representing 79 countries, we found that antibiotic de-escalation occurs in at least half the patients treated for sepsis, usually after 2 to 3 days or longer. De-escalation times roughly paralleled pathogen identification times which were reported to be at least 2 to 3 days in most cases. Cultures were reported to return positive results in approximately half the patients. Rapid diagnostic tests (RDT) were not found to be in wide use, their use being reported by only 28% of survey respondents, and appeared to have similar positive result rates to cultures. The majority of respondents (68%) reported using 2 empiric antibiotics, most commonly, β-lactams/β-lactamase inhibitors, 3rd/4th generation cephalosporins, carbapenems, and vancomycin for Gram-positive coverage. Respondents to the Spanish version of our survey, representing primarily Latin American countries, reported longer culture result times, lower availability of RDTs and a lower rate of antibiotic de-escalation. Multi-drug resistant (MDR) organisms were reported to be fairly common, with about a quarter of respondents reporting these to be “very common.” While MDR rates were very similar between the English and Spanish survey, some countries such as Indonesia, Argentina, Mexico, Romania and India showed a trend towards a significantly higher prevalence of MDR organisms.
Daniel Nichita, MD
References
- Seymour CW, Kahn JM, Martin-Gill C, et al. Delays From First Medical Contact to Antibiotic Administration for Sepsis. Crit Care Med. 2017. 2017 May;45(5):759-765. (PubMed, Article)
- Seymour CW, Gesten F, Prescott HC, et al. Time to Treatment and Mortality during Mandated Emergency Care for Sepsis. N Engl J Med 2017;376:2235-2244. (PubMed, Article)
- Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006 Jun;34(6):1589-1596. (PubMed)
- Klompas M, Calandra T, Singer M. Antibiotics for Sepsis – Finding the Equilibrium. JAMA. 2018;320(14):1433-1434. (PubMed, Article)
- Pulia MS, Redwood R, Sharp B. Antimicrobial Stewardship in the Management of Sepsis. (Article)
- Aslam B, Wang W, Arshad MI, et al. Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist. (PubMed, Article)
- Wolk DM, Johnson JK. Rapid Diagnostics for Blood Cultures: Supporting Decisions for Antimicrobial Therapy and Value-Based Care. The Journal of Applied Laboratory Medicine Jan 2019;3(4):686-697. (Article)
- Schmidt GA, Mandel J. Evaluation and management of suspected sepsis and septic shock in adults. UpToDate Online. Accessed July 18th, 2019. (Article)
- O’Driscoll, Crank CW. Vancomycin-resistant enterococcal infections: epidemiology, clinical manifestations, and optimal management. Infect Drug Resist. 2015;8:217-230. (PubMed, Article)
- Seifi N, Kahani N, Askari E, et al. Inducible clindamycin resistance in Staphylococcus aureus isolates recovered from Mashhad, Iran. Iran J Microbiol. 2012 Jun; 4(2):82-86. (PubMed, Article)
- Bassetti M, Vena A, Croxatto A, et al. How to manage Pseudomonas aeruginosa infections. Drugs Context. 2018;7:212527 (PubMed, Article)
